Biography
He does not have cable, and he subscribes to The Atlantic Monthly. He owns a 1983 Goldwing motorcycle, and his great uncle hid Jews in his barn in the Netherlands during WWII. His favorite molecule is water.
"I have been interested in chemistry since 1960,ŌĆØ said Calvin professor of chemistry Roger DeKock , ŌĆ£What is that? 48 years? ŌĆ” I want to know at the molecular level, ŌĆśHow does that happen?ŌĆÖŌĆØ
DeKockŌĆÖs current research has brought him to that level, where he works with his favorite molecule. ŌĆ£I started to get into water during my ŌĆÖ03-ŌĆÖ04 sabbatical in Calgary,ŌĆØ DeKock said. ŌĆ£Part of my research there dealt with water molecules: hydrogen atoms in aqueous systems can be very mobile.ŌĆØ
Before DeKock earned Fulbright and NATO scholarships for his research, before his lifeŌĆÖs work brought him into contact with prominent social conflicts of the 20th century began, he was a growing boy in Prairie City, Iowa where, ŌĆ£the prairie was there; the city was not,ŌĆØ he remarked. ŌĆ£I was third in my grade school class; there were five of us.ŌĆØ
When it came time for college, DeKock followed the path forged by his older brother: a degree in chemistry from Calvin College. ŌĆ£I thought he was above me,ŌĆØ he said of his model.
Education
- B.A. Calvin College, 1965
- Ph.D. University of Wisconsin, Madison, Wisconsin, Inorganic Chemistry, 1970.
Thesis: Molecular Orbital Calculations for Transition Metal Complexes Containing Pi-Acceptor Ligands
Advisor: Professor Richard F. Fenske
Professional Experience
- Calvin College, 1976-present, Professor of Chemistry
- University of Calgary, Calgary, Alberta, Canada, 2003-2004, Visiting Professor, Department of Chemistry
- Sultan Qaboos University, Muscat, Sultanate of Oman, 1992-1994, Head of Department, Department of Chemistry
- Free University of Amsterdam, Amsterdam, The Netherlands, 1988 Summer,
NATO Research Award, Department of Chemistry - University of California, Berkeley, 1986-1987, Visiting Professor, Department of ChemistryUniversity of Calgary, Calgary, Alberta, Canada, 1985 Summer, Visiting Scientist, Department of Chemistry
- Free University of Amsterdam, Amsterdam, The Netherlands, 1982-83, Fulbright Research Scholar, Department of Chemistry
- University of Notre Dame, Notre Dame, Indiana, 1979-1982 Summers, Visiting Scientist, Department of Chemistry
- American University of Beirut, Beirut, Lebanon, 1972-1976, Assistant Professor of Chemistry, Department of Chemistry
- University of Birmingham, Birmingham, UK, 1970-1972, Imperial Chemical Industries Research Fellow, Department of Chemistry
- University of Florida, Gainesville, Florida, 1969-1970, Postdoctoral Research, Department of Chemistry
Academic Interests
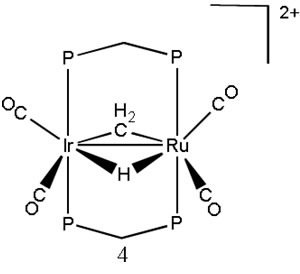
In our research we investigate the structures and relative energies of organometallic complexes that have different placement of hydrogen atoms. In particular we are interested in heterobimetallic complexes (Rh, Os) wherein the hydrogen atom migrates from a metal atom to a bridging methylene ligand. This is part of a larger project that investigates C-H bond activation.
We model molecules using the tools of computational chemistry. Some of our chemical projects are:
1) Hydrogen atom migration in organometallic complexes.
2) Relative energy of molecular and ionic forms of NH3(H2O)7.
3) We are examining methods to extract "effective" nuclear charges from atomic ionization energies.